-
Research Overview
My group is interested in two key research areas to improve the quality of life in individuals affected by genetic disorders which may lead to blindness, and to establish CAR technology in immune cells for specific tumour eradication.
Neuronal degeneration of the retina and retinal pigment epithelium (RPE) in inherited retinal disorder such as retinitis pigmentosa, is the leading cause of blindness worldwide. Recent studies have shown the potential of mesenchymal stem cells (MSC) from various origins in rescuing the visual functions and slowing the progress of degeneration. MSC are multipotent, self-renewing cells that reside mainly in the bone marrow. MSC can also be found in umbilical cord, amniotic fluid, peripheral blood, fallopian tube, cornea, adipose, synovial tissue, dental pulp, placenta, bone, liver and lung.It is believed that MSC are remnants of embryonic stem cells (ESC) that persist in adult life as they exhibit ESC genetic markers of Oct-4, Rex-1, Nanog, GATA-4 and SSEA-1. MSC were hypothesized to be re-distributed via the blood stream to other organs or tissues to maintain stem cells homeostasis in a microenvironment or mobilized upon receiving chemokine signals released by damaged organs and tissues for regenerative purposes. Optimism to use MSC in ocular disorders is based on suggestions that MSC can differentiate into neurons and specifically retinal neurons in vivo following damages caused by chemical induction or burn with laser. Thus, we aim to study and determine the efficiency of MSC to delay degeneration of photoreceptors in an animal model of retinitis pigmentosa (RP).
[caption id="attachment_1306" align="aligncenter" width="300"]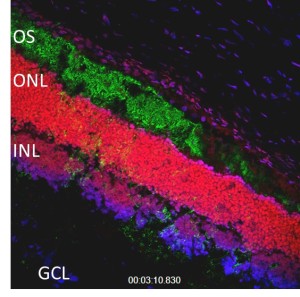 Photoreceptors and nerve layers in the retina[/caption]
Photoreceptors and nerve layers in the retina[/caption]
Adoptive immuno cell therapy for malignancy involves the transfer of ex vivo expanded immune cells as means to augment immune responses to eradicate tumour cells. Few types of immune cells including T-cells have been primed in vitro with cells expressing tumour-specific (TSA) or -associated antigen (TAA) and culture expanded before infusion into autologous receipient. It is hoped that the abundant T-cells in the infusion would be able to launch another sequence of attack on tumour cells. With CAR technology, T-cells would be able to recognize antigen in a similar fashion to B-cells via B-cell receptor (BCR) and could be activated even without presentation by the major histocompatibiliity complex (MHC) molecule by the tumour cells or antigen presenting cells. There are ample researches going on in human trials to target on B cell malignancy, sarcoma, neuroblastoma, prostate cancer, mesothelioma¸ ovarian cancer, head and neck cancer and glioma, and the results have been very promising as the infusion of CAR transduced T-cells were successfully able to induce remission in the cancer patients. Despite the progress in the field of adoptive immune cell therapy using T-cells, such a research has never been carried out in Malaysia and therefore, we would like to take the effort to initiate such research. We aim to transduce a chimeric antigen receptor with specificity to target on CD19 surface marker on B-cell lymphoma and to assess its efficacy in eradicating this tumour cells in both in vitro and in vivo study. B-cell lymphoma is a type of tumour affecting the B-cells in the lymph gland of adults and immunocompromised individual.
Research Funding
Project Title Funding Source Development of a recombinant viral vector to enhance functionality of chimeric antigen receptor Putra Grant Arming T-cells with chimeric antigen receptor (CAR) for B-cell lymphoma Fundamental Research Grant Scheme Selected Publications
CY Wong, SK Cheong, PL Mok & CF Leong 2008 Differentiation of human mesenchymal stem cells into mesangial cells in murine renal glomeruli Pathology 40(1): 56-62PL Mok PL, SK Cheong, CF Leong, KH Chua & O Ainoon 2012 Delivery of erythropoietin in a murine model by nucleofected human adult Marrow Stromal Cells using MIDGE construct Tissue Cell 44(4): 249-56PF Choong, PL Mok, SK Cheong, CF Leong & KY Then 2007 Mesenchymal stromal cell-like characteristics of corneal keratocytes Cytotherapy 9(3): 252-8PL Mok, CF Leong, SK Cheong & O Ainoon 2008 In vitro expression of erythropoietin from transfected human Mesenchymal Stromal Cells Cytotherapy 10(2): 116-24PF Choong, PL Mok, SK Cheong, CF Leong CF & KY Then 2007 Generating neuron-like cells from BM-derived mesenchymal stromal cells in vitro Cytotherapy 9(2): 170-83
Awards
Year Name of Award 2009 Merck Young Scientist Award 2011 1st Prize Poster Presentation. Extended expression of erythropoeitin by MIDGE-transfected human bone marrow mesenchymal stem cells. 9th Malaysian Society of Hematology Meeting, Kuching, Sarawak, Malaysia. 2012 1st Prize Oral Presentation. Subretinal transplantation with human umbilical cord-derived mesenchymal stem. 2nd conjoint (MOH-UKM-USM-UM) Ophthalmology Scientific Conference 2012 ‘ Retina: A Paradigm Shift’, Sunway Pyramid Convention Center, Selangor, Malaysia. 2013 1st Prize Poster Presentation. Establishment of induced pluripotent stem cells from keratinocytes. UTAR National Postgraduate Fundamental and Applied Sciences Seminar 2013 (UTAR NFPASS 2013), Kampar, Perak, Malaysia. 2012 Travel and Conference Award: 8th Asia Pacific Symposium on Neural Regeneration in conjunction with the 5th Pan Pacific Symposium on Stem Cells and Cancer Research & the 1st Cross-Strait Biotechnology Forum (APSNR&PPSSC) Current Lab Members
Name Position Research Interest Year Joined -

Rachel Mok Pooi Ling
[email protected] Gene Therapy